Complete MDR Implementation with 100% Success Rate
![]() Project takeover within 3 days
Project takeover within 3 days
![]() Full quality control by senior consultants in Switzerland
Full quality control by senior consultants in Switzerland
![]() Entire project management and hiring of individual consultants
Entire project management and hiring of individual consultants

Our Clients
Successful completion of Regulatory Projects with a diverse global client base with 100% success rate.




Right First Time. Guaranteed.
Right First Time – as we have conducted many MDR projects, you can rest assured the quality and compliance will be done correctly and efficiently without any extensions or second time round ups.
Our track record has a 100% success rate and we are sure we would achieve the same with you. We guarantee you a successful result.
Full Quality Control
Our senior consultants in Switzerland with a proven track record undertake the full quality control of the project. We have full transparency and the best quality solutions utilising Swiss based Biomedical Engineering Professionals.
Single Point of Contact
Single point of contact for streamlined project management & accountability assurance. You do not need to manage or hire. None of the difficulties of hiring and managing individual consultants
3 Days Project Takeover
Fully remote or on site presence options available. We can takeover the project within 3 days and execute it on priority. We bring faster results than any of the closest competitors.
Competitive Fair Pricing
We have one of the fairest pricing models in the industry, thus enabling us to offer competitive prices for a high quality work.
Fast Track MDR. Guaranteed.
Verification & Validation
What Do Our Clients Say?
A small to medium size medical device company which sell Class I medical devices in the US and Europe (100-500 SKUs) require EU MDR compliance for their devices to continue selling in the European market. An EU authorized representative is already defined; however, several updates are required to the existing procedures (QMS) and product documentation (Tech File) in order to meet the new EU MDR requirements. Swiss MPC resourced the project, updated and created new documents such as Clinical Evaluation, Risk Management and Post Market Surveillance to name a few. In addition, all IFUs and labels were updated to comply with MDR and UDI requirements. Swiss MPC managed the interface with the EU authorized representative until approval was granted.
Timeline 4-6 months
We sought Swiss MPC's help in navigating the MDR transitions. The project management provided by Swiss MPC was exceptional. They know how to match with qualified, capable consultants who have a deep understanding of their field. It was instrumental in meeting and exceeding our goals! Well done!
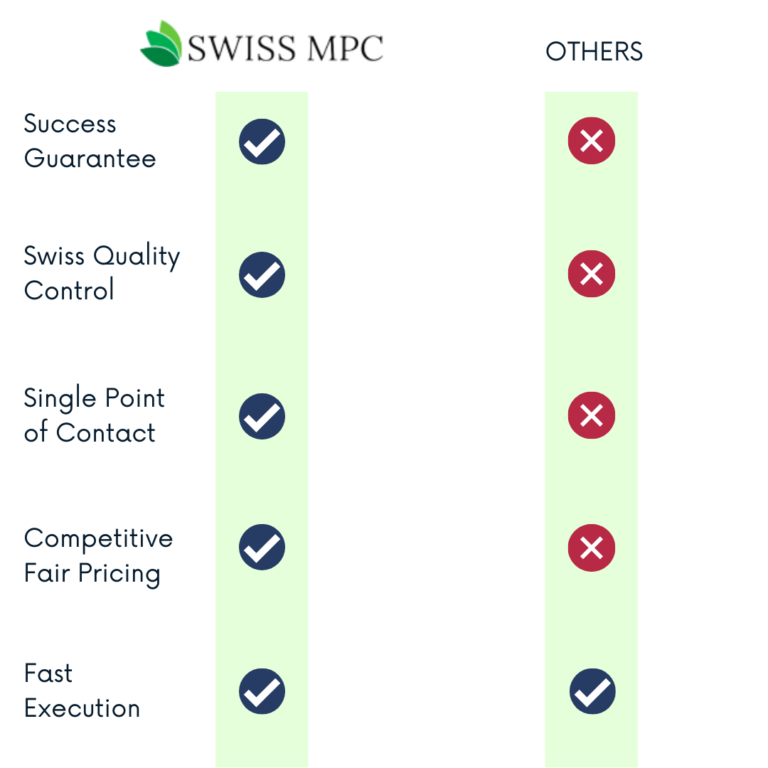
Still Not Sure? Have a look at how we do compared to others
Request More Information
- Receive a meeting invitation to build a tailored Gap Analysis Report
- Receive our detailed case studies
- Receive our team and Consultant details
FAQ
You would receive the following:
Receive a Pre Gap Analysis report tailored to you
Receive our detailed case studies
Receive our team and Consultant details
Yes, our phone numbers are given below but the best way would be to first receive our case studies and team details, so you are already familiar with us and we can directly speak about your project, in the call
The MDR case studies and team details would be sent over email.
The team briefs can be found in the about section.
We are a European Team based out of Switzerland and Ireland.
We offer remote and on site working possibilities.
We have a thorough experience with MDR and a 100% success rate. We are confident, if we take your project, you would see successful results and hence offer a guarantee on it too.