Complete DHF Implementation with 100% Success Rate
![]() Staffing from 0 to 10 FTEs within 3 weeks
Staffing from 0 to 10 FTEs within 3 weeks
![]() Full quality control in Switzerland
Full quality control in Switzerland
![]() Remediation for legacy product compliance to new standards
Remediation for legacy product compliance to new standards

Our Clients
Successful completion of Regulatory Projects with a diverse global client base with 100% success rate.




Right First Time. Guaranteed.
Right First Time – as we have conducted many remediation projects, you can rest assured the quality and compliance will be done correctly and efficiently without any extensions or second time round ups.
Our track record has a 100% success rate and we are sure we would achieve the same with you. We guarantee you a successful result.
Full Quality Control
Our senior consultants in Switzerland with a proven track record undertake the full quality control of the project. We have full transparency and the best quality solutions utilising Swiss based Biomedical Engineering Professionals.
Experts For Each Area
Experts for each respective area, for example: Risk Management, Verification and Validation, Biocompatibility, Regulatory compliance, etc.
Dedicated team, tailored to your project, built to minimize disruption of internal resources
3 Days Project Takeover
Fully remote or on site presence options available. We can takeover the project within 3 days and execute it on priority. Staffing from 0 to 10 FTEs within 3 weeks
Competitive Fair Pricing
We have one of the fairest pricing models in the industry, thus enabling us to offer competitive prices for a high quality work.
Fast Track MDR. Guaranteed.
Verification & Validation
What Do Our Clients Say?
A global pharmaceutical company required technical support with second sourcing drug primary packaging components as well as medical devices which are intended to be used with a drug product (combination product). This involved a landscape evaluation of the market, the acquisition of samples, lab testing, machinability testing and ultimately official technical and DHF documentation to support the newly introduced components with existing drug products.
Timeline 24-36 months
A global orthopedic company specializing in implants and instruments (Class IIa and Class III) required DHF updates to remediate legacy and new systems to comply with new global regulations. Swiss MPC supported determining manufacturing adjuvants and their impact on biocompatibility as well as IFU and Labelling changes. In addition, many DHF documents were reviewed or updated.
Timeline 12-15 months
We sought Swiss MPC's help in navigating our DHF situation. The project management provided by Swiss MPC was exceptional. They know how to match with qualified, capable consultants who have a deep understanding of their field. It was instrumental in meeting and exceeding our goals! Well done!
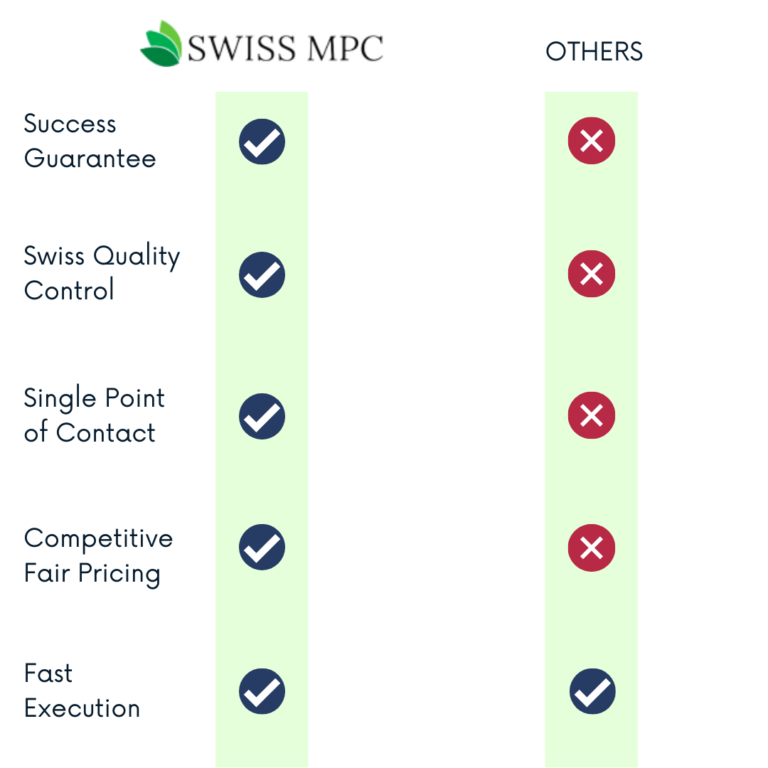
Still Not Sure? Have a look at how we do compared to others
Request More Information
- Receive a meeting invitation to build a tailored Gap Analysis Report
- Receive our detailed case studies
- Receive our team and Consultant details
FAQ
Yes, our phone numbers are given below but the best way would be to first receive our case studies and team details, so you are already familiar with us and we can directly speak about your project, in the call
The MDR case studies and team details would be sent over email.
The team briefs can be found in the about section.
We are a European Team based out of Switzerland and Ireland.
We offer remote and on site working possibilities.
We have a thorough experience with DHF and a 100% success rate. We are confident, if we take your project, you would see successful results and hence offer a guarantee on it too.